Incyte Corporation
CEO : Mr. Herve Hoppenot
Quarterly earnings growth(YoY,%)
| Period | Revenue | Operating Income | EPS | Release Date |
|---|---|---|---|---|
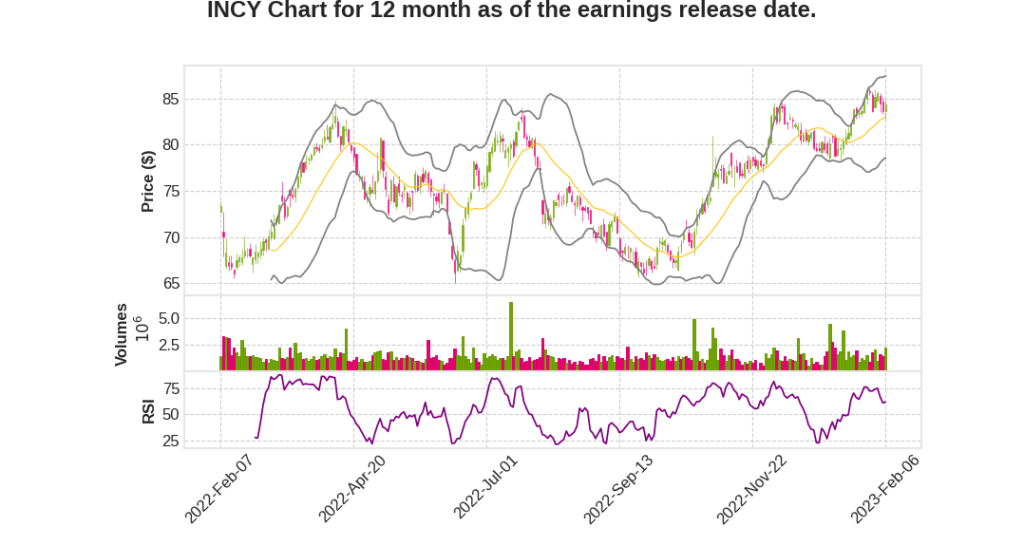
| 2022 Q4 | 7.4% YoY | -36.7% | -94.9% | 2023-02-07 |
Steven Stein says,
Expected regulatory and clinical updates
- The PDUFA date for ruxolitinib XR is March 23, 2023, and the expected approval is an important step towards fixed-dose combinations with parsaclisib, zilurgisertib and the BET inhibitor.
- More mature data sets of ruxolitinib with ALK2 and BET are expected in the second half of 2023. Depending on the data, pivotal trials could be started with one or both of these compounds.
- Pivotal data midyear from AGAVE-201, evaluating axatilimab in third-line chronic graft versus host disease is expected.
- More mature data set of small molecule oral PD-L1 program 280 and 318 are expected in the second half of 2023
- Initiation of combination trials of 280 with adagrasib, CTLA-4, and an oral VEGF inhibitor in the first half of 2023 is planned.
- Phase 2 data in both vitiligo and prurigo nodularis from povorcitinib are expected later this year.
- IL-15 receptor beta monoclonal antibody, auremolimab, is expected to enter the clinic for vitiligo.
Key clinical and regulatory achievements
- Initial results of the Phase 1/2 study evaluating the ALK2 inhibitor zilurgisertib demonstrated improvement in anemia and hemoglobin responses in patients with myelofibrosis.
- Discovery of 989, a novel anti-mutant calreticulin monoclonal antibody that selectively inhibits the proliferation and differentiation of cells harboring mutant CALR.
- Opzelura gained its second indication in vitiligo.
- Multiple Phase 2 studies initiated in different conditions, including lichen planus, lichen sclerosis, and hidradenitis suppurativa, as there are no topical or oral therapies approved.
Early pipeline development
- INCB123667, a novel potent and selective oral small molecule inhibitor of CDK2 entered Phase 1 clinical development.
- Anti-mutant CALR monoclonal antibody will enter the clinic this year.
Financial update
- Financial update was not discussed in this speech.
Barry Flannelly says,
Jakafi’s Strong Performance
- Jakafi net sales grew 9% YoY to $647 million in Q4 and 13% for the full year to $2.4 billion
- Total patient demand rose 7% in 2022
- The launch of Jakafi in chronic GVHD continues to be strong with the total number of patients in Q4 growing 11% versus prior year quarter
- Average duration of therapy is approximately 15 months, driving growth in GVHD
- Expect strong growth in 2023 with full year net product revenues to be between $2.53 billion to $2.63 billion
Opzelura’s Significant Growth Potential
- Opzelura had a strong quarter with continued double-digit sequential growth in patient demand in atopic dermatitis and a very successful launch in vitiligo
- Total demand grew 34% in Q4 versus prior quarter to reach 84,700 units, driven by both new patient growth and an increasing number of refills
- Total full year net sales for Opzelura were $129 million
- Opzelura is the #1 prescribed branded agent for new AD patients and its impact on itch remains unmatched by any other topical therapy
- Potential approval in pediatric AD next year for two to 11-year olds
- The size of the market and the potential opportunity for Opzelura in vitiligo is substantial with an estimated 1.5 million patients diagnosed with vitiligo in the U.S.
- The approval of Opzelura in vitiligo in Europe is expected in the next few months, where there are an estimated 1.5 million diagnosed patients living with the disease
Monjuvi, Minjuvi and Pemazyre
- Monjuvi sales in Q4 were $24 million, up 13% year-over-year, and revenues were $89 million for the year
- Net sales for Minjuvi were $20 million for the full year
- Pemazyre grew to $83 million in net sales in 2022 with $20 million coming from outside the U.S.
Q & A sessions,
Opzelura Sales Team Focus
- Sales team focused on educating healthcare professionals for vitiligo and atopic dermatitis
- Concentrating on reinforcing compliance and need for Opzelura for a longer period of time
- Concentrating on ensuring patients get refills for atopic dermatitis
Coverage and Launch Information
- Commercial patients with insurance access is 84%, coverage beyond that is 90%
- Medicaid coverage in all 50 states, commercial insurance coverage improving
- Opzelura coverage for AD and vitiligo are essentially the same
- RUX QD launch planned after PDUFA date in March, announces approval in April
GVHD Patient Population and Competition
- 1,500 patients in steroid-refractory acute GVHD
- 14,000 patients in chronic GVHD, most growth coming from chronic GVHD
- Physician population for resistant population in chronic GVHD is much larger
- Competition from ibrutinib and Rezurock, but axatilimab opportunity is great
ALK2 and BET Programs
- ALK2 program showed hemoglobin increases and expects to make pivotal decisions on populations to go after by end of year
- BET program will continue to dose escalate and likely go after suboptimal responders in pivotal study
Pediatric Atopic Dermatitis and Povorcitinib
- Pediatric atopic dermatitis study addresses a population from 2 to 11 years of age, efficacy expected to be the same as in adults, and no unusual safety issues expected
- Povorcitinib indication for patients with body surface area involvement of 8% or above, every expectation of substantial efficacy, and safety labeling will be dealt with at the end
Lumber Program and RUX+Parsaclisib Study
- Trial ongoing for suboptimal responders in ruxolitinib, with final Phase 2 data showing encouraging spleen and symptom response
- Additionally, safety profile in MF looks very clean thus far, and Phase 3 is set up to be positive
- First-line study will take longer to read out, and is an all-comer standard in terms of endpoints