Charles River Laboratories International, Inc.
CEO : Mr. James C. Foster J.D.
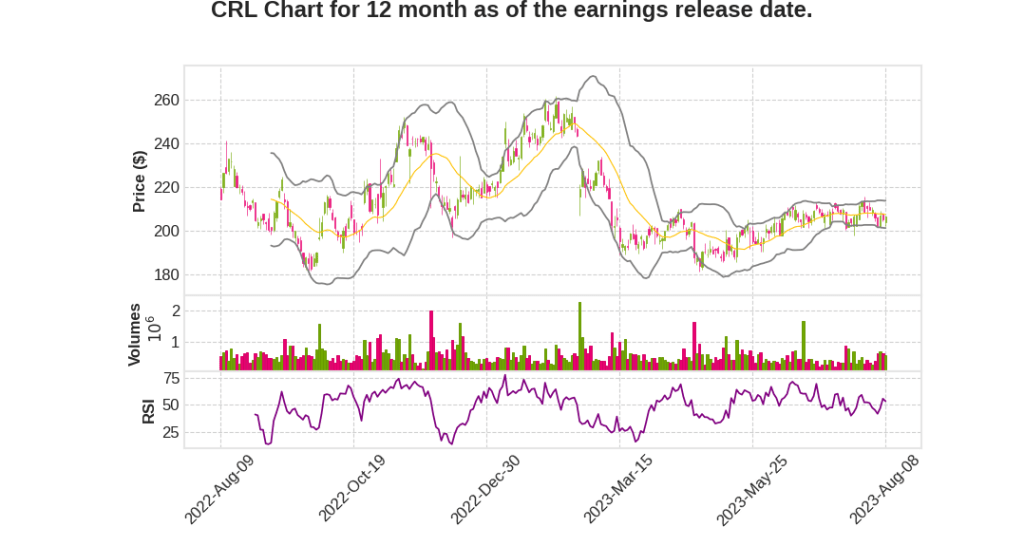
Quarterly earnings growth(YoY,%)
| Period | Revenue | Operating Income | EPS | Release Date |
|---|---|---|---|---|
| 2023 Q2 | 8.9% YoY | 11.6% | -12.1% | 2023-08-09 |
Jim Foster says,
Revenue Growth and Guidance
- Organic revenue growth in Q2 2023 was 11.2%, with improvement seen in the RMS and Manufacturing segments.
- Revenue growth rates in the DSA segment remained strong, with low double-digit growth and improved operating margins.
- Revenue growth in the second half of 2023 is expected to be pressured primarily in the DSA segment due to lower backlog and booking trends, challenging year-over-year comparisons, and modest impact from NHP supply constraints.
- Biopharmaceutical clients are reprioritizing their pipelines and tightening their R&D budgets, which may impact demand trends.
- Non-GAAP earnings per share guidance for 2023 has been narrowed to the upper end of the previous range.
NHP Supply and Mitigation Efforts
- The company has successfully mitigated the logistical challenges posed by NHP supply constraints by conducting more studies outside of the U.S. and leveraging their global infrastructure.
- Progress has been made in study scheduling, logistics, quarantine operations, and retraining of staff at international sites.
- No significant NHP supply constraints are expected to affect the business in Q4 2023 and next year.
- The impact from NHP supply constraints is now expected to be less than the initial outlook of a 2%-4% impact to consolidated revenue growth this year.
Q2 2023 Performance
- Revenue in Q2 2023 was $1.06 billion, an 8.9% increase over the previous year.
- Organic revenue growth of 11.2% was driven by strong performance in the RMS and DSA segments, as well as improvement in the manufacturing growth rate.
- Global biopharmaceutical clients outpaced small and mid-sized biotech clients, demonstrating the diversity and stability of the overall client base.
- Operating margin was 20.4%, a decrease of 140 basis points year-over-year, primarily due to margin pressure in the manufacturing segment and higher unallocated corporate costs.
- Earnings per share were $2.69, a decrease of 2.9% from the previous year.
Revenue and Non-GAAP Earnings Guidance
- Organic revenue growth guidance for 2023 has been narrowed to a range of 5.5% to 7.5%.
- Non-GAAP earnings per share guidance for 2023 has been narrowed to a range of $10.30 to $10.90.
- The lower end of the guidance ranges has been increased by 50 basis points for organic revenue growth and $0.40 per share for earnings.
- Existing DSA backlog and assessment of demand trends give confidence in achieving financial outlook for the year.
Segment Performance: DSA
- DSA revenue in Q2 2023 was $663.5 million, an 11.7% increase on an organic basis.
- Safety Assessment business drove DSA revenue growth, with contributions from base pricing, study volume, and small benefit from NHP pricing.
- Discovery services business posted lower revenue due to the current market environment and shorter-term nature of projects and backlog.
- DSA backlog decreased modestly on a sequential basis, primarily due to higher cancellation rates as clients rationalize lower priority projects in their pipeline.
- Gross bookings remain robust, supporting healthy revenue growth rates once cancellation rates decline.
- DSA margin was 27.6%, a 230 basis point increase from Q2 2022, driven by operating leverage associated with higher revenue in the Safety Assessment business.
Segment Performance: RMS
- RMS revenue in Q2 2023 was $209.9 million, a 13.9% increase on an organic basis.
- RMS segment benefited from broad-based growth in all geographic regions, with exceptional performance from the end-sourcing solutions business.
- Timing of large model shipments in China and stable demand and pricing for small research models in North America and Europe contributed to growth.
- Services businesses, particularly in-sourcing solutions, drove growth, with CRADL operations expanding and generating client interest.
- RMS operating margin increased by 150 basis points to 26.4%, primarily due to leverage from higher revenue growth in China.
James Foster says,
Enhanced Centers of Excellence
- The company now has three centers of excellence in gene-modified cell therapy manufacturing, viral vector manufacturing, and plasmids.
- This change from the acquired companies is aimed at improving the overall operations and expertise.
Improved Staffing and Books of Business
- The company has upgraded staffing across the board, including sales, regulatory, and general management.
- There is an improvement in the books of business in all three areas of focus.
- However, the time frame for generating new business may vary across these areas.
Transition to Commercial Domain
- The company is in discussions with a few clients to move into a commercial domain in the cell therapy manufacturing business.
- It may take some time for the market to acknowledge the company’s presence and take their products and services seriously.
Confidence in Higher Growth Rates
- Despite a challenging year of retooling, the company is confident in achieving higher growth rates this year.
- Meaningful improvement is expected compared to the previous year.
Hopes for Top Line Growth and Margin Improvement
- The company has high hopes for significant top line growth, which would benefit both manufacturing and overall company performance.
- Improvements in margins are anticipated as the scale of operations improves.
Q & A sessions,
Backlog and Slippage
- Clients booked studies far in advance due to concerns about getting slots
- Current backlog is around thirteen months, with a reduction in slippage expected
- Studies are expected to be booked closer to when they start
Business Performance
- First half of the year was strong, but second half may be less strong
- Demand for studies is solid, with a strong competitive situation
- Testing for biologics is expected to improve in the back half of the year
- CDMO business is improving on both the top and bottom line
Pricing and Volume
- Price will continue to be an important factor given the complexity of the work
- Capacity is expected to be tight, with a busy worldwide basis
- Access to capital and IPO markets will impact cash flow
New Modalities and Franchises
- The microbial business is strong, with potential for growth
- CDMO business is improving, while the biologics business is anticipated to improve in the second half of the year
- Cell and gene therapies are a focus, with multiple clients and commercial opportunities
Market Conditions and Client Base
- Market conditions are difficult to gauge due to different factors each cycle
- Clients are favoring the clinic over early discovery work
- Client base includes both small biotech companies and big pharma companies